
VOLUME 64 : 2016
VOLUME 64 : 2016
ACTA MANILANA publishes research and innovation in the different branches of the natural and applied sciences. It reports significant development in the discipline, and novel applications, unconfined by the traditional coverage of the disciplines.
Pulse electrodeposited tin/palladium/exfoliated graphene oxide (Sn/Pd/EGO) catalyst for direct ethanol fuel cell applications
Page 41–50
Paulo Irvin A. Chang, Joshua L. Tan, & Bernard John V. Tongol
ARTICLE DOI: https://doi.org/10.53603/actamanil.64.2016.ykng6739
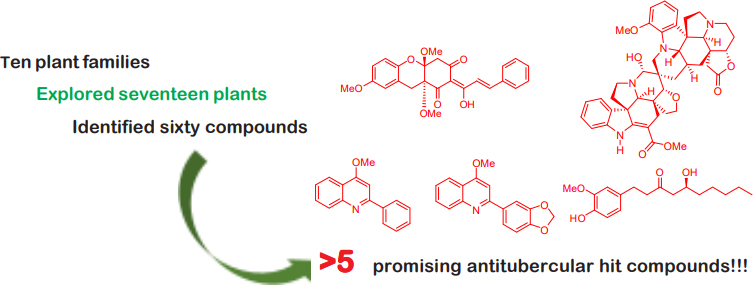
Graphical Abstract
Abstract
This study aims to utilize pulse electrodeposition to synthesize tin/palladium/exfoliated graphene oxide (Sn/Pd/EGO) catalyst composite as anode material for ethanol oxidation reaction (EOR) which finds significant application in Direct Ethanol Fuel Cell (DEFC) application. The electrocatalytic activity of the prepared catalysts towards ethanol oxidation was evaluated using cyclic voltammetry in 1 M ethanol in 0.1 M NaOH. Cyclic voltammetry showed that the addition of Sn to Pd improved the catalytic activity of Pd towards EOR in alkaline solution. The bimetallic Sn/Pd catalyst on glassy carbon electrode (Sn/Pd/GCE) showed a higher catalytic activity with a peak current density of 28.1 mA cm-–2, compared to Pd/GCE (23.39 mA cm–2). The electrocatalytic activity of bimetallic Sn/Pd catalyst further improved when EGO was used as a support (Sn/Pd/EGO) with a peak current density of 36.7 mA cm–2. Atomic force microscopy revealed that the catalysts were deposited on the edges of the graphene sheets forming bright clusters with dark fringes on the middle of the structure. X-ray photoelectron spectroscopy confirmed the presence of Pd and Sn metals and their oxides. This study demonstrates that pulse electrodeposition could offer a much simpler and faster approach in the development of high performance catalysts for EOR.
Keywords: direct ethanol fuel cells, graphene, Sn/Pd, AFM, XPS, pulse electrodeposition
FOLLOW US
-
Research Center for the Natural and Applied Sciences
Thomas Aquinas Research Complex Building
University of Santo Tomas España, 1015 Manila, Philippines -
TL: (+63 2) 3406-1611 local 4037
DL: (+63 2) 8731-4031 - actamanilana@ust.edu.ph

© 2021 University of Santo Tomas, Acta Manilana. All rights reserved
Powered by: Communications Bureau